

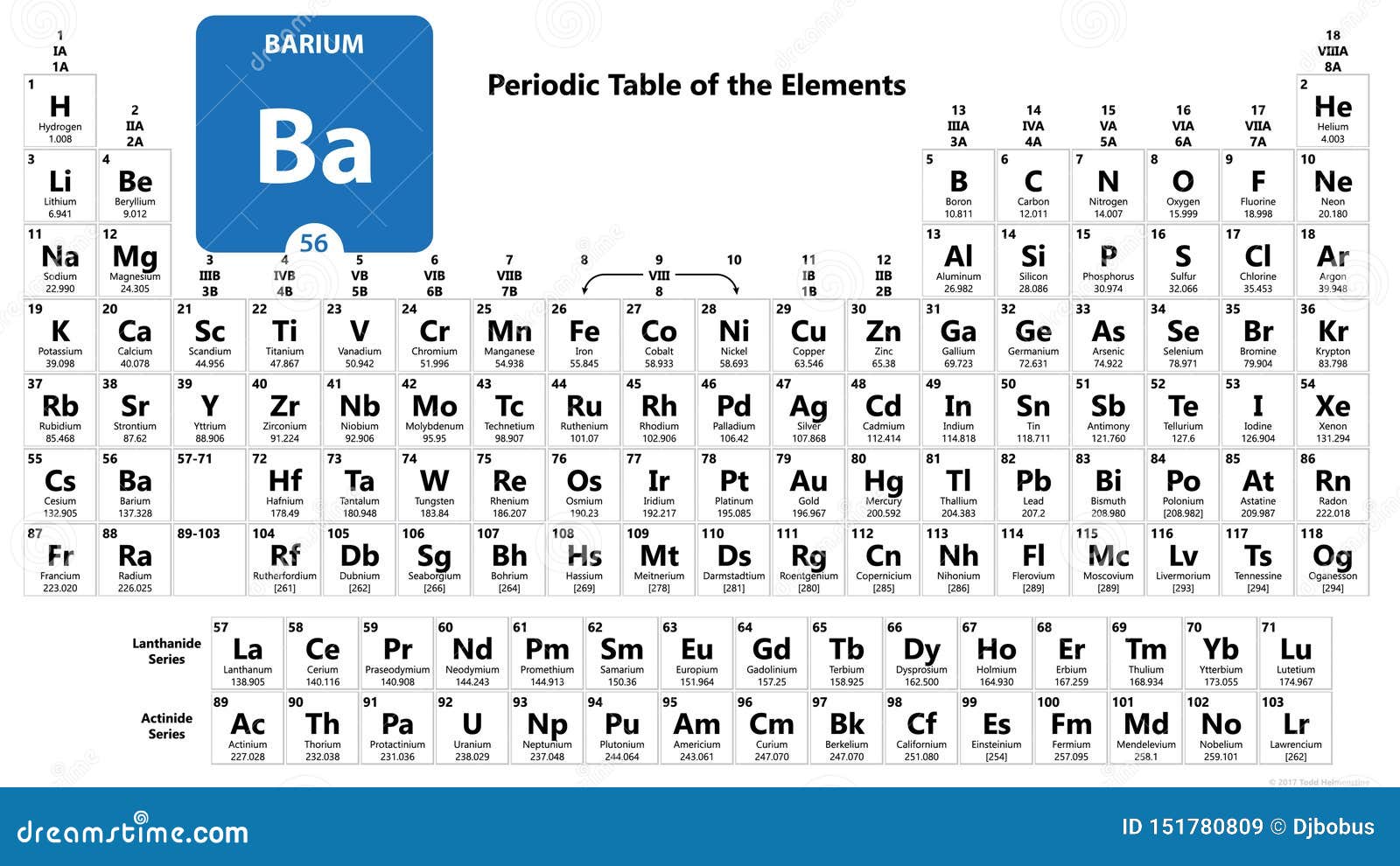
The chemical elements are what the periodic table classifies and organises. Each distinct atomic number therefore corresponds to a class of atom: these classes are called the chemical elements. We also produce Barium as rod, ingot, pieces, pellets, disc, granules, wire, and in compound forms, such as oxide. This is called the atomic number, often symbolised Z (for 'Zahl' German for 'number'). We can also provide many materials in the nanoscale range. Our standard powder particle sizes average in the range of - 325 mesh, - 100 mesh, 10-50 microns and submicron (< 1 micron). Barium Particles are also available as Nanoparticles. Atomic Number: 56: Atomic Weight: 137.327: Element Type: Alkali Earth Metal: Crystal Structure: Cubic Body Centered: Melting Point: 727.0☌ 1340.6☏ 1000. The seven rows of the table are called a periodic table. Current trends in particle usage or in development include commercialization of technologies such as rapid solidification and metal injection molding and production of dense powder metallurgy products. It is a tabular display of chemical elements, arranged in order of atomic number in rows so that elements with similar atomic structure and recurring chemical properties appear in vertical columns. Total number of protons in the nucleus is called the atomic. Metal particle powders are used in a variety of applications including, additives in paint and other coatings, in solid fuels and cements, as pigments in printing and packaging and dietary supplements in food processing. Barium is a chemical element with atomic number 56 which means there are 56 protons in its nucleus. Thin Film Deposition & Evaporation MaterialsĪmerican Elements specializes in producing high purity Barium Particles with the smallest possible average grain sizes for use in preparation of pressed and bonded sputtering targets and in Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD) processes including Thermal and Electron Beam (E-Beam) Evaporation, Low Temperature Organic Evaporation, Atomic Layer Deposition (ALD), Metallic-Organic and Chemical Vapor Deposition (MOCVD).Additive Manufacturing & 3D Printing Materials.Therefore, a barium atom has fifty-six protons and fifty-six electrons. The atomic number of an element is equal to the number of protons and electrons in that element. Barium is the 56th element of the periodic table so its atomic number is 56. This periodic filling of the electron shells results in a periodic variation of properties of atoms as you increase in atomic number. Barium is a classified alkaline earth metal and its symbol is ‘Ba’. Helium will have 2 K electrons, lithium will have 2 K electrons and 1 L electron, beryllium will have 2 K electrons and 2 L electrons, boron will have 2 K electrons and 3 L electrons, and so forth. This makes it easier to remove the outer electron(s) from the atom. Since the number of electrons is responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. Are the numbers of electrons per orbital also just be an average or a probability. Therefore hydrogen (whose atomic number is 1) will only have one electron in the first shell, or 1K electron. Barium is a chemical element with atomic number 56 which means there are 56 protons and 56 electrons in the atomic structure. The first inner shell (known as the K shell) has two positions for electrons, the next shell (the L shell) has 8. 4 This nuclide decays by double electron capture (absorbing. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Naturally occurring barium ( 56 Ba) is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, identified as being unstable by geochemical means (from analysis of the presence of its daughter xenon-130 in rocks) in 2001. This is because the inner shells are of lower energy than the outer ones. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.
These electrons usually fill the lower shells (orbits) first. Thought of another way, the electrons orbit the atoms nucleus in different sized orbits, with some closer to the nucleus and some further away.Īs you increase the atomic number of the atom, you increase the number of electrons in orbit around the nucleus. The electrons can be considered to sit in energy levels or shells, dictated by the quantum energy of each electron. Electrons exist in separate orbitals or energy states surrounding the nucleus. The rare-earth ions most often replace the alkaline earth atoms that are close in ionic radius and coordination number. Protons have a positive(+) charge, neutrons have no charge and electrons have a negative(-) charge. Protons and Neutrons are very much larger than electrons.


 0 kommentar(er)
0 kommentar(er)
